Water consists of one oxygen atom and two hydrogen atoms: H2O. Atoms trend to have eight electrons in their outer spheres. Hydrogen, like all elements of the 1. Period of the periodic system of elements, is an exception. It has only one electron and one sphere, but it trends to have two electrons. One could say that hydrogen atoms are constantly trying to add another electron to their spheres.
Oxygen atoms have two electrons in their inner sphere and six on their outer one but trend to have eight outer sphere electrons. One could say that oxygen atoms are constantly trying to add another two electrons to their outer spheres. When one oxygen atom combines with two hydrogen atoms, the two single electrons on the spheres of the two hydrogen atoms are added to the outer sphere of the oxygen atom. The result of this combination is that the oxygen atom ends up with its ideal eight electrons on its outer sphere and the two hydrogen atoms end up with two electrons.
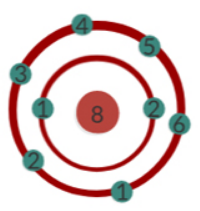


It is easy to see that, if two hydrogen atoms attach themselves to one oxygen atom, the oxygen atom gets its ideal number of electrons on its outer sphere: eight. Simultaneously, the two hydrogen atoms now have two electrons each instead of one. This is why hydrogen and oxygen are such a perfect match.
In this combination of two hydrogen atoms with one oxygen atom the oxygen atom attracts the electrons more strongly than the hydrogen atoms do. According to the periodic system of elements, oxygen has an electron negativity of 3.5 and hydrogen of 2.2. Therefore, the electrons are not evenly distributed in the water molecule.
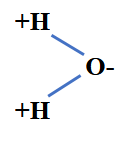
The water molecule assumes a triangular shape and is more positively charged on the side where the hydrogen atoms are located and more negatively on the oxygen side.
This makes water molecules behave like little magnets, each with a positive and a negative pole. As we all know, positive and negative magnetic poles attract each other, while negative and negative and positive and positive poles reject each other. Water molecules must therefore line up and form chains, each molecule moving its positive pole as close as possible to the negative poles of other water molecules. This is called dipole interaction.
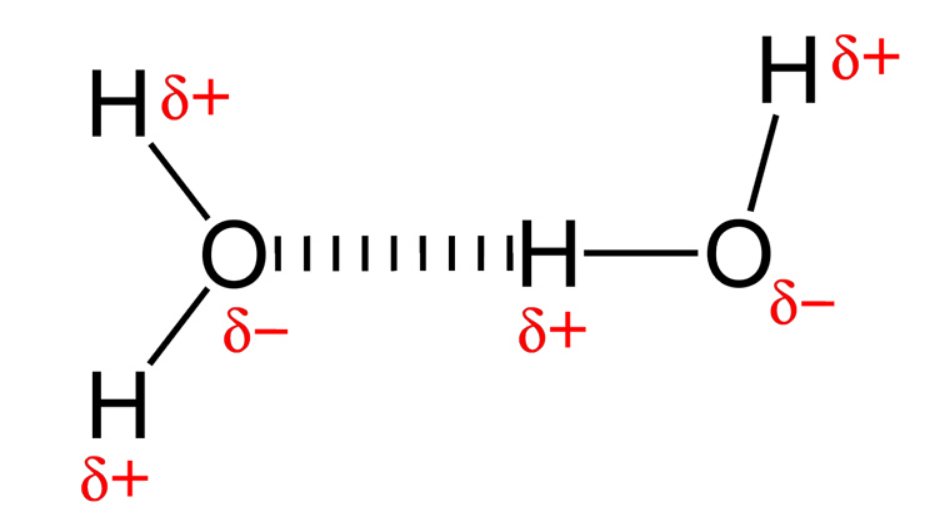
The direct effect of dipole interaction is called cohesion, the property of molecules to stick together. Cohesion is the cause of surface tension of water surfaces, which allows the surface of liquids, in particular of water, to contract to the smallest possible surface area.
The strong cohesion between water molecules is responsible for the ability of water to form drops like raindrops or drops on leaves. It is also the cause of the strong adhesion of water to other substances, which enables water to “climb” upward against gravity in capillary tubes.
This capillary effect in turn enables water to ascend in high trees from the deepest roots to the highest branches and leaves. Think about it: Sequoia trees are about 300 feet high plus maybe 100 feet for the roots. For such a tree to live, water must be transported up 400 feet high or higher against gravity. Due to its dipole, water adheres to other surfaces better than any other liquid. This makes it possible for plants to transport it upward. In other words: without the dipole effect of water plant life on earth would not be possible. And without plant life there probably would not be any animal and human life on earth either. It is not exaggerated to say that probably without the dipole effect of water no life would have developed on earth.
If energy is removed from substances, their molecules contract and the density of the substance increases. Only water reacts differently. The cohesive force of the dipole water molecules is responsible for what is called the “density anomaly of water”. After it shrinks initially when it freezes, water expands again below 4 degrees Celsius. This is not only the cause of busted water pipes in cold winters, it is also the cause why even during the coldest periods of geo-history water never turned completely into ice. When water reaches 4 degrees Celsius it sinks under the ice because of its greater density and higher specific weight. This makes it impossible for large lakes or other large water bodies to freeze up completely. Some water under the ice remains and makes the continuation of life possible. We can see why life originated from water.
Since the magnetic forces of the water dipole cause it to adhere to other substances, water is also a super solvent, which changes the density properties of all water-soluble matter. This gives water a critical role in all metabolism processes. Water dipoles are superconductors and capable of transmitting electrical impulses, which are generated in the context of perception – seeing, hearing, feeling, tasting, smelling. These impulses are conducted and transmitted via the inner cells, which contain mainly water. And our bodies are 70% water, too.
We can state with confidence that without the water dipole there would be no life on earth – at least not the kind of life we know and of which we are a part. Scientists and movie makers have speculated that life might also be based on ammonia instead of on water, because ammonia is also a dipole molecule. However, it is much weaker than the water dipole and it does not have the density anomaly of water. It is theoretically possible though that perhaps on another planet a form of ecosystem and life exists that is based on the ammonia dipole. It would be very different from our water-based life and incompatible with it.
As for me, I find it hard to believe that the water dipole is pure accident. In my view, this intelligent detail strongly suggest that it has been designed by an extremely smart chemical engineering mind.
It’s a Great Outstanding Design.

